• Alan A. Lowe, DMD, Dip. Ortho., PhD, FRCD(C) •
© J Can Dent Assoc 1999; 65:571-4
[Appliance Development |Features of Klearway Appliance|Suggested Klearway Appointment Sequence |Conclusion |References]
The American Sleep Disorders Association recommends that oral appliances (OAs) be used in patients with primary snoring or mild obstructive sleep apnea (OSA) and in patients with moderate to severe OSA1,2 who are intolerant of or refuse treatment with nasal continuous positive airway pressure (nCPAP). Because of the life-threatening implications of a number of sleep disorders, it is imperative that OA therapy commences only after a complete medical assessment. For some patients, combination therapy with other treatments such as weight loss, surgery and nCPAP may be indicated, and such combination therapy must be coordinated by the attending sleep physician.
Oral appliance design variations may have a direct effect on treatment outcome for a specific patient, and the advantages and disadvantages of each design vary considerably. For more complete overviews of the range of appliances currently used, the reader is directed to previously published reviews.3-5
The Snore Guard appliance is a prefabricated OA lined with a soft, thermosensitive material that moulds to the patient’s teeth. The Snore Guard engages the mandible mainly at the incisors; therefore, it applies the force of advancement to fewer teeth than appliances that utilize full occlusal coverage. The advantages of the Snore Guard are its relatively low cost and reduced chair time. The disadvantages are that it is nonadjustable, it may apply excessive pressure to the lower anterior teeth in some patients and retention problems may develop over time. Ferguson and others compared the efficacy, side effects, patient compliance and preference of four months of Snore Guard and nCPAP therapies in a randomized, prospective, crossover study in patients with mild to moderate OSA.6 Forty-eight per cent of the patients who used the Snore Guard were treatment successes, 24% were compliance failures (unable or unwilling to use the treatment) and 28% were treatment failures. Four people refused to use nCPAP after using the Snore Guard. Sixty-two per cent of the patients who used nCPAP were overall treatment successes, 38% were compliance failures, and there were no treatment failures. Side effects were more common with nCPAP, and the patients were less satisfied. Seven patients were treatment successes with both treatments; six of these patients preferred Snore Guard, and one preferred nCPAP as a long-term treatment. The authors concluded that Snore Guard was an effective treatment in some patients with mild to moderate OSA and is associated with fewer side effects and greater patient satisfaction than nCPAP.
The Tongue Retaining Device (TRD) is a custom-made appliance with an anterior bulb that holds the tongue in a forward position during sleep by means of negative pressure. Currently, the TRD appears to be the appliance of choice for patients who have few or no teeth and for patients who have large tongues. In addition, the TRD is a good appliance for patients who cannot adequately advance their mandible for whatever reason. Its disadvantages are that it is more difficult for both clinicians and patients to use on a regular basis, it poses problems for patients who cannot breathe through their nose, and it is a single jaw and tongue position appliance. The effects of the TRD on tongue muscle activity have been investigated in two reports. Ono and others evaluated the effect of a TRD on awake genioglossus (GG) muscle activity in adult OSA subjects and in symptom-free control subjects matched by age and by body mass.7 It was concluded that the TRD has different effects on the awake GG muscle activity in control subjects and patients with OSA. In a subsequent study, Ono and others evaluated the effect of a TRD on sleep-state GG muscle activity in seven OSA subjects.8 The TRD was found to reduce the OSA severity, normalize the time lag and counteract fluctuating GG activity observed when no bulb was present.
Clinicians soon identified a need for an OA that could be adjusted and did not require a series of remakes if the initial jaw position was not adequately positioned forward. An adjustable appliance with a hinge positioned lingual to the lower incisors that allowed progressive advancement of the mandible was evaluated by Ferguson and others in 1997.9 Of the patients who used the adjustable OA, 55% were treatment successes, 5% were compliance failures and 40% were treatment failures. Several disadvantages were noted with this initial adjustable appliance. Many of the patients could not tolerate the 1.5-mm incremental advancements dictated by the design of this initial adjustable appliance, retention problems were observed, and it was thought that the lingually located hinge mechanism possibly encroached on tongue space and subsequently on airway size in some of the patients.
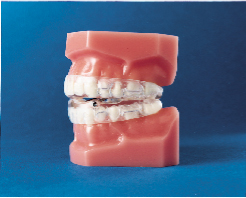
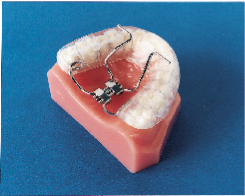
Subsequently, the Klearway appliance (Figs. 1 and 2) was designed to increase retention, to remove the hinge apparatus from behind the lower incisors (to avoid encroaching on tongue space) and to incorporate 44 0.25-mm increments of mandibular advancement to improve patient tolerance. Lowe and others evaluated the effect of the Klearway appliance on the respiratory disturbance index (RDI) and airway size in a series of 38 OSA patients recruited at three sites across Canada.10 The mean RDI before treatment was 32.6; after the insertion of the Klearway appliance, the RDI was reduced to 12.1. RDI was reduced to less than 15 in 80% of a group of moderate OSA patients (RDI 15 to 30) and in 61% of a group of severe OSA patients (RDI > 30). Covert compliance data measured with a newly developed, miniaturized, temperature-sensitive monitor embedded in the appliance indicated that it was worn for a mean of 6.8 hours per night. Using awake videoendoscopy at end tidal expiration during quiet nasal breathing in the supine position, Ryan and others documented that the size of the hypopharynx and velopharynx increased with the Klearway appliance in place, but no significant differences in the oropharynx could be identified.11 The lateral diameter of the velopharynx increased more than the anteroposterior dimension.
Features of the Klearway Appliance
Klearway is effective because it keeps the teeth together and holds the lower jaw and tongue forward during sleep to open the airway. It possesses excellent retention characteristics designed to keep the appliance in the mouth during all the various complex jaw movements that can occur during sleep. It provides full occlusal coverage of both arches and is designed not to encroach on tongue space (Figs. 1 and 2). Furthermore, it facilitates the slow and gradual movement of the mandible by permitting the patient to adjust the appliance according to his or her own comfort level with the guidance of the attending dentist.
This fully adjustable OA is much more comfortable to wear than a single-jaw-position appliance, which often may require time-consuming and expensive remakes to place the mandible in the ideal forward position required to open the airway adequately. Fabricated of thermoactive acrylic, Klearway becomes pliable for easy insertion and conforms securely to the dentition for an excellent fit while significantly decreasing soft tissue and tooth discomfort. Forty-four forward positions are available in increments of 0.25 mm, which covers a full 11.0-mm range of antero-posterior movement. Such small increments help avoid the rapid forward jaw movements that can cause significant patient discomfort.
Klearway allows the patient to feel less restricted and thus less claustrophobic — a sensation experienced by a small number of patients during the first few nights of wear. Once the appliance is warmed under hot water and inserted, the acrylic resin hardens as it cools to body temperature and firmly affixes itself to both arches. Lateral and vertical jaw movement is permitted, which enables the patient to yawn, swallow and drink water without dislodging the appliance. Patients with bruxism are also very comfortable with this appliance since it does not prevent jaw movements during sleep.
Suggested Klearway Appointment Sequence
After referral from the patient’s sleep physician, the dentist is encouraged to follow the protocol developed by the Sleep Disorders Dental Society. Patients with mild temporomandibular joint discomfort or bruxism or both can usually wear Klearway with ease, since the jaw position used is very comfortable for both the joint and the dentition over the long term. Totally edentulous patients may not be ideally suited for treatment with mandibular repositioners, because they may not have enough intraoral retention to keep the appliance in the mouth during sleep; a TRD should be considered in such cases. Patients with edentulous maxillary arches and adequate teeth in the lower arch may respond favourably to Klearway therapy. A stone model of both arches from impressions is submitted to the laboratory together with a bite registration taken at two-thirds the distance from centric occlusion to full protrusion. For the best accuracy, a George gauge with a grey 2.0-mm fork should be used for the bite registration. Specific upper and lower teeth (first bicuspids and molars unless otherwise requested) are selected for the placement of ball or Adam’s clasps (or both) in the event that added retention may be needed later in treatment.
After showing the patient the custom-made appliance at the insertion appointment, describe all the possible limitations and side effects of this form of therapy. Answer any questions, and have the patient read and sign an informed consent form before inserting the appliance. Instruct the patient to insert the appliance by first submerging it in a container of hot tap water or by holding the appliance under running water that is only as hot as the fingers can comfortably tolerate. Instruct the patient not to heat the appliance by any other means, as it could be irreversibly damaged. Have the patient look in a mirror while inserting the appliance into the mouth and pressing the upper rim up onto the upper back teeth. Once the appliance is fully seated on the upper teeth, have the patient close the lower teeth forward into the lower portion of the appliance and bite firmly. To remove the appliance, the patient first rinses the mouth with warm water. Then the patient is to grasp the edges of the upper back portion and pull down (not forward) to dislodge the appliance from the upper jaw. The patient then pushes up on the edges of the lower rim with both thumbs while opening the mouth. Instruct the patient not to remove the appliance by simply opening the mouth, because the wire work may be permanently distorted.
To clean the appliance, the patient should use a stiff toothbrush with any toothpaste. The tooth portion of the appliance, the smooth outside surface and the expansion screw should all be brushed thoroughly. Because this appliance is made from thermoacrylic material, it is not necessary to keep the appliance soaking in water or mouthwash during the day. Advise the patient to use a cleanser tablet to help remove stains and to keep the appliance fresh.
At the one-week follow-up appointment, record the amount of opening of the expansion screw with a Boley gauge or a millimetre ruler. If the patient experiences significant jaw discomfort, turn the screw in the reverse direction of the arrow to decrease the amount of mandibular protrusion until the patient is comfortable. The appliance is designed in the laboratory to allow for this setback from the initial two-thirds forward position if it is required after the initial insertion. Relieve the acrylic around any sore or uncomfortable teeth. A sense of the teeth not touching completely may be experienced by some patients in the morning. This usually disappears within an hour or so. In addition, they may experience an excessive amount of saliva for the first month or so.
After one month of regular wear, record again the amount of opening of the expansion screw with a Boley gauge or a millimetre ruler. If the patient is still snoring, if the bed partner witnesses apneic events or if the patient still feels sleep deprived, instruct the patient to activate the appliance by turning the screw on the top of the appliance two times per week until the next appointment. Each turn or activation in the direction of the arrow will move the lower jaw gradually forward in 0.25-mm increments. Have the patient insert the tip of the key into the hole on the side of the expansion screw at the base of the arrow and then turn or push the key toward the direction of the arrow imprinted in the metal expansion screw. The arrow shows the correct movement to advance the lower jaw. Once the key is completely turned from one side to the other, the patient removes it, and a new hole appears for the next turn. Turning the key opposite to the direction of the arrow closes the expansion screw and retracts the mandible. If significant jaw or joint discomfort occurs, advise the patient to stop turning the screw until the next visit. If the discomfort has not subsided in one or two days, have the patient call the office immediately.
Some patients stop snoring and feel more rested shortly after Klearway is inserted, and no further advancement of the mandible is required. Others may require two or three months of slow and gradual forward repositioning before a significant treatment effect is noted. When the patient and bed partner report a cessation of snoring and a resolution of symptoms, further advancement of the mandible may not be required and the appliance is considered to be titrated. The expansion screw should be tied off with stainless steel ligature wire or filled in with cold-cure acrylic to prevent any further movement of the mandible. Expansion screws may self-close over time and therefore should always be permanently stabilized for long-term Klearway wear. The patient should be referred back to the physician or sleep specialist for assessment at this time.
Recall appointments should be scheduled every six months. At each appointment, check the status of the occlusion and verify that the appliance has not been distorted. Minor cracks in the appliance can be repaired at the chair side with cold-cure acrylic. The overall management of the patient’s particular sleep disorder remains the responsibility of the attending physician.
The Klearway appliance is the most extensively researched appliance available today. It has been shown to effectively increase airway size, to be worn consistently and to have a significant effect on both snoring and OSA.
The treatment of snoring and OSA can be a very exciting and rewarding part of any dental practice. Improving the quality of someone’s life with an OA can significantly alter a practitioner’s perspective on health care delivery. An international group of dentists with expertise in this area, the Sleep Disorders Dental Society, provides a newsletter, an annual meeting, a slide set and manual, and a library for member dentists. The Society also has a resource centre for use by both patients and dentists (telephone 724-935-0836).a
Acknowledgment: Klearway was invented by the author at the University of British Columbia. International patents have been obtained by the university and specific licencees are assigned the rights to manufacture and distribute the appliance worldwide.
Dr. Lowe is professor and chair, division of orthodontics, department of oral health sciences, faculty of dentistry, University of British Columbia.
Reprint requests to: Dr. Alan A. Lowe, Division of Orthodontics, Department of Oral Health Sciences, Faculty of Dentistry, The University of British Columbia, 2199 Wesbrook Mall, Vancouver, BC V6T 1Z3
Research undertaken by the author and discussed in this article was supported by grants from the Medical Research Council of Canada, the British Columbia Lung Association and the National Centres of Excellence, Inspiraplex.
1. Schmidt-Nowara W, Lowe A, Wiegand L, Cartwright R, Perez- Guerra F, Menn S. Oral appliances for the treatment of snoring and obstructive sleep apnea: a review. Sleep 1995; 18:501-10.
2. American Sleep Disorders Association Standards of Practice Committee. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances. Sleep 1995; 18:511-3.
3. Lowe AA. Dental appliances for the treatment of snoring and/or obstructive sleep apnea. In: Kryger M, Roth T and Dement W, eds. Principles and practice of sleep medicine. 2nd edition, W.B. Saunders Co., 1994. p. 722-35.
4. Lowe AA and Schmidt-Nowara WW. Oral appliance therapy for snoring and apnea. In: Pack A, Lenfant C, eds. Pathogenesis, diagnosis and treatment of sleep apnea. Marcel Dekker Inc., In Press, 1999.
5. Lowe AA. Oral appliances in obstructive sleep apnea. In: Kryger M, Roth T and Dement W, eds. Principles and practice of sleep medicine. 3rd edition. W.B. Saunders Co., In Press, 1999.
6. Ferguson KA, Ono T, Lowe AA, Keenan SP, Love LL, Wiggs, B and Fleetham JA. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest 1996; 109:1269-75.
7. Ono T, Lowe AA, Ferguson KA, P E, Fleetham JA. The effect of the tongue retaining device on awake genioglossus muscle activity in patients with obstructive sleep apnea. Am J Orthod Dentofacial Orthop 1996; 110:28-35.
8. Ono T, Lowe AA, Ferguson KA, Fleetham JA. A tongue retaining device and sleep-state genioglossus muscle activity in patients with obstructive sleep apnea. Angle Orthod 1996; 66:273- 80.
9. Ferguson KA, Ono T, Lowe AA, al-Majed S, Love LL, Fleetham JA. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnea. Thorax 1997; 52:362-8.
10. Lowe AA, Sjöholm TT, Ryan CF, Fleetham JA, Ferguson KA, Remmers JR. Treatment, airway and compliance effects of a titratable oral appliance. Sleep In Press, 1999.
11. Ryan CF, Love L, Peat D, Fleetham J, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnea: effect on awake calibre of the velopharynx. Thorax In Press, 1999.